With nearly seven million Americans currently living with Alzheimer’s disease — and 13 million projected to have the illness by 2050 — early diagnosis and treatment are more urgent than ever.
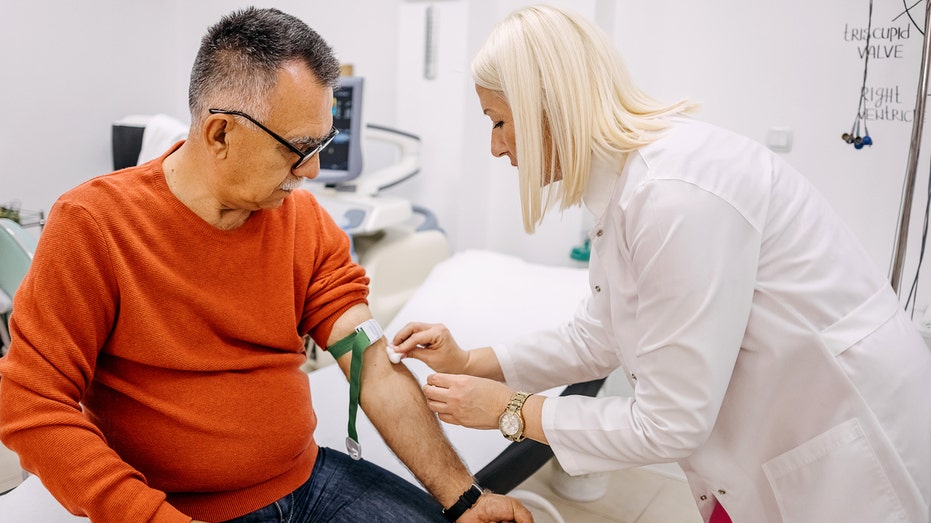
To help address this, Mayo Clinic has announced a new, non-invasive blood test that detects a protein in the brain that signals Alzheimer’s.
The goal, doctors say, is for this test to offer a convenient, less invasive alternative to traditional diagnostic methods.
‘REVERSING’ ALZHEIMER’S: HERE ARE EXERCISES TO MAKE THE BRAIN MORE RESILIENT
Fox News Digital spoke to Dr. Alicia Algeciras-Schimnich, professor of laboratory medicine and pathology at Mayo Clinic Rochester in Minnesota, about the new test and what it means for Alzheimer’s patients and their families.
“This is the first Alzheimer’s disease blood test offered at Mayo Clinic Laboratories,” said Algeciras-Schimnich, who led the clinical validation study to gauge how well the test performed.
“While there are other commercial blood tests for Alzheimer’s disease, the uniqueness of our test is its high accuracy rate.”
One of the hallmark features in the brains of patients with Alzheimer’s disease is the formation of plaque containing a protein known as beta amyloid.
“The pTau217 assay assesses the accumulation of beta amyloid in the brain by measuring the amount of phosphorylated Tau 217 (p-Tau217) in the test sample,” said Algeciras-Schimnich.
EXPERIMENTAL ALZHEIMER’S DRUG GETS FDA ADVISORY PANEL’S THUMBS-UP: ‘PROGRESS IS HAPPENING’
Accumulation of beta amyloid in the brain can also be evaluated by imaging techniques, such as PET scans or cerebrospinal fluid (CSF) biomarkers, the doctor noted — but those methods have some limitations.
“The PET scan to evaluate beta amyloid is expensive and not a widely available technology,” said Algeciras-Schimnich.
“And the CSF collection requires an invasive technique to remove spinal fluid, so it is also not widely used.”
The Alzheimer’s disease blood biomarkers serve as a non-invasive tool that can improve access for patients who need answers, she said.
In patients with symptoms of cognitive decline, the blood test has a sensitivity of 92% and a specificity of 96%.
“Sensitivity measures the ability of the test to correctly identify patients with the disease, while specificity measures the ability of the test to correctly identify those without the disease,” Algeciras-Schimnich explained.
Patients who take the test are classified as positive or negative for the presence of the accumulation of beta amyloid.
“In a small number of patients, the test will not be able to differentiate between the presence or absence of beta amyloid,” said Algeciras-Schimnich.
“These patients will need additional tests to determine if they are positive or negative for the accumulation of beta amyloid.”
The test is purposely designed to minimize the number of false positive results as compared to other tests, the doctor said.
“The test has been validated at Mayo Clinic through a rigorous quality process backed by scientific experts and clinicians,” Algeciras-Schimnich said.
The test is currently available for clinicians to order through Mayo Clinic Laboratories.
“Since it is a blood test, it requires a blood draw by a phlebotomist, so anyone who is averse to blood should be aware,” said Algeciras-Schimnich.
At this point, the test is only recommended for individuals 50 years of age and older who have symptoms of mild cognitive impairment or mild dementia.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“We don’t yet have enough data to support how the test performs in younger individuals,” Algeciras-Schimnich said.
Michelle Rankine, PhD, a certified dementia practitioner in Texas, is not associated with Mayo Clinic but shared her comments on the test’s potential.
“As the global burden of Alzheimer’s continues to rise, a blood-based test not only offers convenience, but could help transform Alzheimer’s disease research,” Rankine told Fox News Digital.
“This could make screening more efficient in averting the clinical onset of Alzheimer’s disease.”
For more Health articles, visit www.foxnews/health
“This innovation addresses a growing need and could accelerate development of new treatments, improve patient evaluation and care, and potentially even allow for early intervention before symptoms become worse,” Rankine added.
Article Source: Health From Fox News Read More