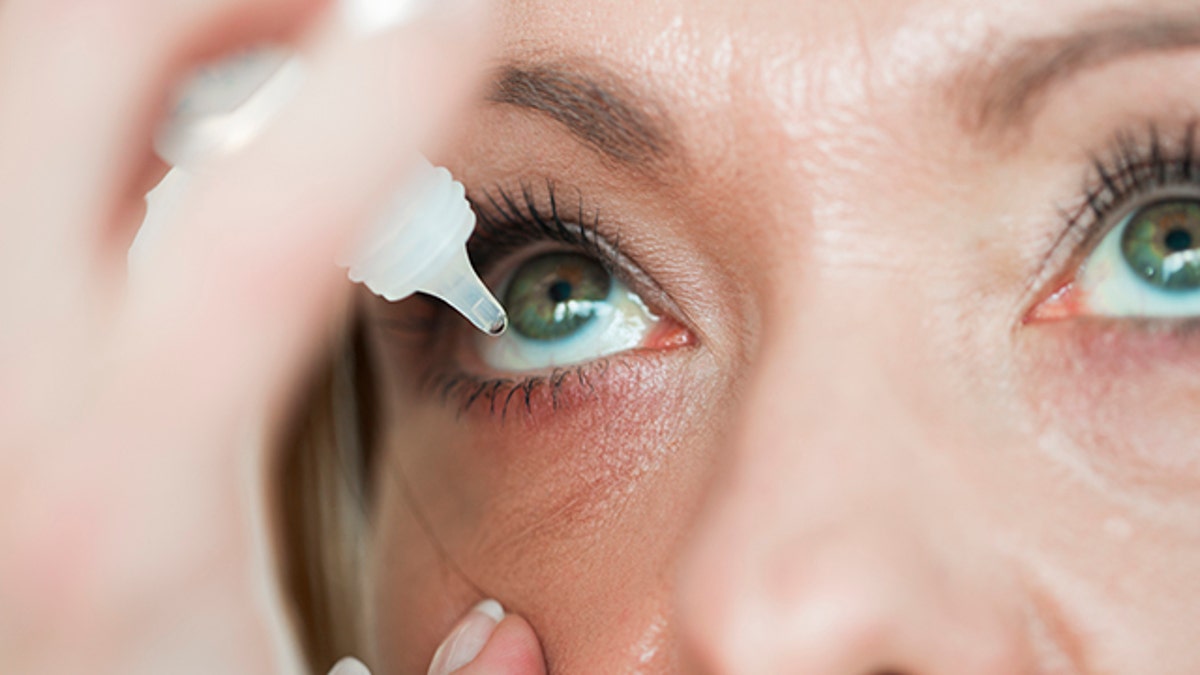
U.S. officials recently reported two more deaths and additional cases of vision loss linked to eye drops tainted with drug-resistant bacteria — with the CDC identifying the issue now in 16 states, including California, New York, Illinois, Texas and Pennsylvania.
Most cases have been linked to four regional clusters. EzriCare’s drops are the only product used by patients in each group, the Associated Press reported.
The eye drops from EzriCare and Delsam Pharma were recalled in February — and health authorities are continuing to track infections as they investigate the outbreak.
EYE DROPS AND SAFETY ISSUES: HERE’S WHAT YOU NEED TO KNOW NOW
The recalled drops were manufactured by Global Pharma Healthcare in India, where the bacteria — Pseudomonas aeruginosa — is commonly linked to outbreaks in hospitals.
Dr. James Kelly, M.D., an ophthalmologist based in New York City and founder of Kelly Vision Center, appeared on “Fox & Friends Weekend” on Saturday to discuss the outbreak of the drug-resistant bacteria linked to eye drops.
“The investigation is ongoing,” said Kelly on Saturday morning.
“So the contamination where sterility is compromised — it could have happened anywhere, from the manufacturing to the packaging to the handling to the storing. At any of those points, contamination can occur.”
He added, “I don’t think it’s known yet. So we will eventually find out.”
EYE DROP RECALL: FLORIDA WOMAN SUES COMPANY AFTER EYE REMOVED
The doctor also said, “Unfortunately, we never think of eye drops as being something that could cause this type of [infection or] have lethal consequences. In this particular instance, the bacteria [are] very drug-resistant bacteria.”
He also described the bacteria as “very aggressive.”
Kelly reiterated, “Where the contamination happened, no one’s really clear about that.”
Even so, he said there are specific measures that people at home can take to decrease the chances of contamination spreading or harming them.
“Before you use eye drops, wash your hands,” said Kelly.
FDA WARNS OF BACTERIAL CONTAMINATION IN INDIAN MANUFACTURER’S EYE DROPS
He also said, “Inspect the bottle, actually. Look for any visible contamination or within the liquid contents of the bottle.”
Then, he said, once the bottle of eye drops is uncapped, people should not allow “the tip of the bottle” to touch or come in contact with “any surface.”
Instead, said Kelly, “Just allow the drop to go directly into [the] eye.”
Then, he said, promptly “recap it and store it in a clean, dry location.”
EYE SPY A BIG PROBLEM: CALIFORNIA DOCTOR REMOVES 23 CONTACT LENSES FROM ONE WOMAN’S EYE
The doctor also said that “expiration dates matter. We know that the effectiveness of a medication is less after the expiration date. The risk of contamination is higher — so look at the expiration date on your medicine, especially eye drops.”
Kelly also said that anyone who is experiencing “any kind of pain, light sensitivity, blurry vision or discharge” should “seek immediate medical care.”
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
To learn more, watch the video at the top of this article, or click here to access it.
The Associated Press contributed to this report.
Article Source: Health From Fox News Read More