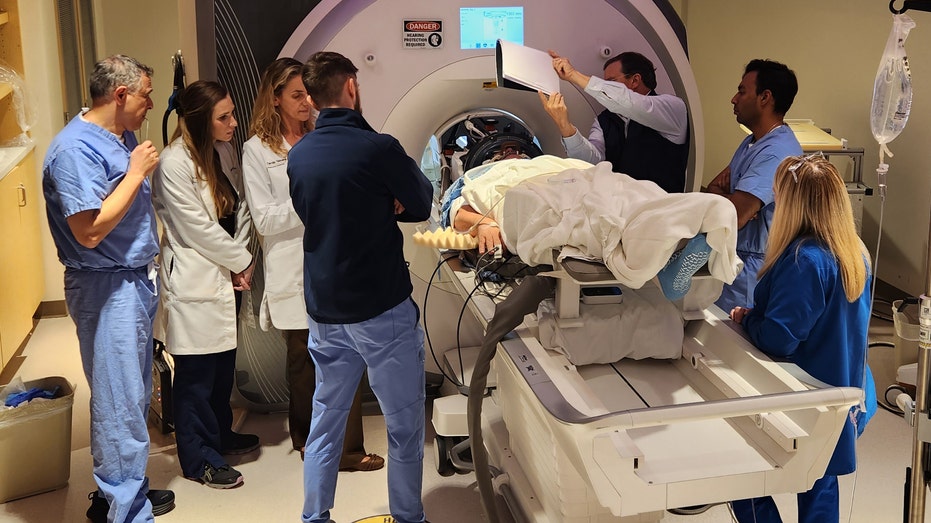
A new Alzheimer’s therapy has shown potential in the first human trials.
Researchers at the West Virginia University Rockefeller Neuroscience Institute (RNI) found that by pairing focused ultrasound in combination with antibody therapies, they were able to accelerate the removal of amyloid-beta plaques from the brains of patients with Alzheimer’s disease.
The study findings were published in The New England Journal of Medicine on Jan. 11.
FASTING COULD REDUCE SIGNS OF ALZHEIMER’S DISEASE, STUDIES SUGGEST: ‘PROFOUND EFFECTS’
An abnormal buildup of amyloid-beta proteins is one of the hallmarks of Alzheimer’s, as these proteins clump together to form plaques that interfere with neurons in the brain.
Anti-amyloid-beta monoclonal antibody treatments, such as aducanumab and lecanemab, have proven to be effective in clearing these plaques and slowing disease progression.
But until now the drugs have been limited by the blood-brain barrier (BBB), which is designed to keep harmful substances from reaching the brain, according to a press release from RNI.
More than 98% of drugs are blocked by the barrier, which means patients require higher doses and more frequent therapies, the researchers noted.
ALZHEIMER’S BLOOD TEST COULD HIT THE MARKET IN EARLY 2024, RESEARCHERS SAY
In this study, scientists used a focused ultrasound (FUS) system to temporarily open the blood-brain barrier, which allowed the antibodies to have greater access to areas of the brain with high amyloid-beta plaques.
After six months of antibody treatment, the study participants had an average of 32% more reduction in amyloid-beta plaques in areas where the BBB was opened compared to areas where the drug was used without the ultrasound, the release stated.
“This was a first in human safety and feasibility study in three participants demonstrating that the BBB opening can accelerate clearance of beta amyloid plaques,” study lead Dr. Ali Rezai, director of the Rockefeller Neuroscience Institute (RNI) at WVU, told Fox News Digital.
“Non-invasive focused ultrasound is an outpatient procedure that allows for targeted delivery of therapeutics to the brain that can potentially accelerate the benefit of the antibody treatment in Alzheimer’s disease,” he added.
DEMENTIA AMONG YOUNGER PEOPLE IS LINKED TO 15 FACTORS, MAJOR STUDY REVEALS
The three patients, between the ages of 59 and 77, all had mild Alzheimer’s disease.
During the study, they received six monthly infusions of the aducanumab antibody.
After each treatment, the focused ultrasound was used to open the BBB at the sites of the highest plaque buildup.
While there are some potential risks associated with ultrasound use, such as brain swelling and hemorrhage, Rezai said those effects were not observed in this study.
“We verified with MRI scans that the BBB opening was temporary and it closed 24 to 48 hours after the FUS procedure,” he told Fox News Digital.
The reductions in amyloid plaques were verified in PET scans.
SMOKING SHRINKS THE BRAIN AND DRIVES UP ALZHEIMER’S RISK, NEW STUDY FINDS
This was the first step toward larger studies; in those, researchers will be able to evaluate more patients and larger areas of the brain, Rezai noted.
In the next phase of the clinical trial, the ultrasound therapy will be paired with lecanemab, another anti-beta amyloid antibody.
Dr. James Galvin, director of the Comprehensive Center for Brain Health at UHealth, the University of Miami Health System, was not involved in the WVU research but shared his reaction.
“A study like this is important because it demonstrates that there may be safe ways to increase drug delivery to the brain without any serious adverse effects,” he told Fox News Digital.
DEMENTIA’S STAGGERING FINANCIAL COST IS REVEALED IN NEW REPORT: IT’S ‘BANKRUPTING FAMILIES’
“Focused ultrasound has been used in other treatment paradigms for brain diseases such as Parkinson’s disease and brain tumors,” Galvin went on.
Galvin also cautioned that this research was conducted with only three patients and was not a placebo-controlled study.
“It was also designed as a safety study and not appropriately powered to detect significant clinical changes,” he added. “It is still too early to make any specific recommendations, but I am excited to see if there are planned follow-up studies with a larger number of patients.”
Rebecca M. Edelmayer, PhD, senior director of scientific engagement at the Alzheimer’s Association, was also not involved in the study but called the results “very intriguing,” albeit preliminary.
“The blood brain barrier, in its healthy form, protects the brain from harmful agents that could reach it via the bloodstream,” she told Fox News Digital via email.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“Getting therapeutics across this barrier — from the bloodstream into the brain tissue — is a challenge for any drug used to treat brain diseases, including drugs to treat Alzheimer’s disease.”
Edelmayer added that while this was a “very small study of relatively short length,” it was a worthwhile way to test a “cutting-edge idea” for improving the effectiveness of Alzheimer’s medications.
Focused ultrasound-induced blood-brain barrier opening has also been shown to improve drug delivery to treat brain tumors, Edelmayer pointed out.
“This is a great example of how learnings from research in other diseases might be repurposed for Alzheimer’s disease and other dementia.”
Looking ahead, Edelmayer said the results of this early research point to the need for larger-scale, longer trials.
“We need more research in individuals with Alzheimer’s disease from all communities to know the full impact this approach could have.”
For more Health articles, visit www.foxnews.com/health.
Article Source: Health From Fox News Read More